Background
Essential thrombocythemia (ET) is a myeloproliferative neoplasm (MPN) associated with an increased risk of thrombotic events (TEs), which represent a substantial cause of mortality in this population. There is limited contemporary, real-world evidence exploring the effect of TEs on mortality in patients with ET. The aim of this analysis was to compare risk of mortality among patients newly diagnosed with intermediate- or high-risk ET who experienced a TE vs those who did not experience a TE.
Study Design and Methods
All data from the Medicare Fee-for-Service (FFS) claims database (Parts A/B/D) from January 2010-December 2017 were used to identify patients with an ET diagnosis (all intermediate or high risk based on cohort age ≥65 years) with ≥1 inpatient or ≥2 outpatient claims. The index date was the date of the first qualifying ET claim. Patients with an ET diagnosis, use of hydroxyurea, or use of ruxolitinib within 12 months before the index date and patients with a myelofibrosis diagnosis during the study period were excluded. A minimum of 12 months of continuous medical and pharmacy enrollment pre-index was required. The study sample was categorized into TE and non-TE groups based on the occurrence of any of the following events during follow-up: deep vein thrombosis, pulmonary embolism, ischemic stroke, acute myocardial infarction, transient ischemic attack, peripheral arterial thrombosis, or superficial thrombophlebitis. TEs were evaluated from the index date to the end of follow-up. Cox regression analyses with time-varying effects were used to assess mortality risk among patients with ET with post-index TE as a time-dependent variable, stratified by pre-index TE, and adjusting for patient demographic characteristics and comorbid conditions.
Results
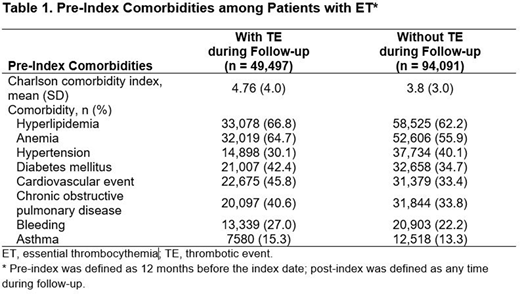
A total of 143,588 Medicare FFS beneficiaries with a diagnosis of ET met the study inclusion criteria; median age was 76.0 years, 68.1% were female, and 84.2% were white. Pre-index TE was reported in 37,284 patients (26.0%). In the follow-up period, 49,497 patients (34.5%) had a TE and 94,091 patients (65.5%) did not have a TE. In the comparison between the TE vs non-TE groups, the median (range) age (77 [65-107] vs 75 [65-110] years, respectively), mean (SD) Charlson comorbidity index score (4.8 [3.5] vs 3.8 [3.5]), and percentage of patients with a history of bleeding (27.0% vs 22.2%), anemia (64.7% vs 55.9%), or a cardiovascular event (45.8% vs 33.5%) were higher (Table 1).
The median time from ET diagnosis to first TE in the follow-up period was 2.8 months for all patients, 0.5 months for patients with pre-index TE, and 8.0 months for those without pre-index TE. The most common types of first TE in the follow-up period were ischemic stroke (37.3%), acute myocardial infarction (23.2%), and transient ischemic attack (22.6%).
The risk of mortality was increased for patients who experienced a TE compared with those who did not (hazard ratio [HR; 95% CI], 11.2 [10.6-11.8]; P<0.001), including for both those with pre-index TE (HR [95% CI], 9.3 [8.6-10.1]; P<0.001) and those without pre-index TE (HR [95% CI], 15.4 [14.2-16.8]; P<0.001).
Conclusions
In this contemporary, real-world analysis, approximately one-third of patients with newly diagnosed intermediate- to high-risk ET experienced a TE. Elderly patients with ET who experienced a TE had an approximately 11-fold increased risk of mortality vs those who did not experience a TE, highlighting a continued unmet need in this population. Further efforts are needed to better define and mitigate TE risk in patients with ET, particularly in those with prior TE.
Pemmaraju:Daiichi Sankyo: Research Funding; Cellectis: Research Funding; Plexxikon: Research Funding; Blueprint Medicines: Honoraria; AbbVie: Honoraria, Research Funding; Samus Therapeutics: Research Funding; Affymetrix: Other: Grant Support, Research Funding; Roche Diagnostics: Honoraria; Stemline Therapeutics: Honoraria, Research Funding; Pacylex Pharmaceuticals: Consultancy; Incyte Corporation: Honoraria; Novartis: Honoraria, Research Funding; LFB Biotechnologies: Honoraria; MustangBio: Honoraria; Celgene: Honoraria; DAVA Oncology: Honoraria; SagerStrong Foundation: Other: Grant Support. Gerds:Gilead Sciences: Research Funding; Incyte Corporation: Consultancy, Research Funding; Roche/Genentech: Research Funding; AstraZeneca/MedImmune: Consultancy; Imago Biosciences: Research Funding; Sierra Oncology: Research Funding; Celgene: Consultancy, Research Funding; Apexx Oncology: Consultancy; CTI Biopharma: Consultancy, Research Funding; Pfizer: Research Funding. Yu:Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. Parasuraman:Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. Shah:Avalere Health: Current Employment. Xi:Incyte Corporation: Other: Avalere Health is a paid consultant of Incyte Corporation; Avalere Health: Current Employment. Kumar:Incyte Corporation: Other: Avalere Health is a paid consultant of Incyte Corporation; Avalere Health: Current Employment. Scherber:Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. Verstovsek:CTI Biopharma Corp: Research Funding; Protagonist Therapeutics: Research Funding; Roche: Research Funding; Incyte Corporation: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; PharmaEssentia: Research Funding; Sierra Oncology: Consultancy, Research Funding; Blueprint Medicines Corp: Research Funding; Genentech: Research Funding; AstraZeneca: Research Funding; ItalPharma: Research Funding; Promedior: Research Funding; Gilead: Research Funding; Celgene: Consultancy, Research Funding; NS Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.